Event
Inquires please contact Camille Pride at campride@sas.upenn.edu
Title: Elucidating Photochemistry of Fluorescent Protein based Biosensors with Femtosecond Raman Spectroscopy
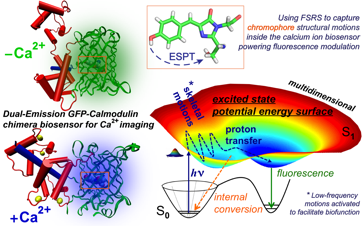
Abstract: Imaging calcium ions (Ca2+) in living systems holds great potential to revolutionize neuroscience, cancer research, and cellular biology. The recent development of genetically encoded Ca2+ sensors for optical imaging (GECO) has expanded the color palette of calcium ion sensors with improved fluorescence properties.1 Though directed evolution with random mutagenesis has been useful in generating new fluorescent protein based biosensors, the mechanistic understanding of fluorescence mechanisms is required to enable targeted design of biosensors with desired properties. We use femtosecond stimulated Raman spectroscopy (FSRS)2,3 to investigate two GECOs: a green-blue dual-emission ratiometric GEM-GECO1 with a serine-tyrosine-glycine chromophore4,5 and a green intensiometric G-GECO1.1 with a threonine-tyrosine-glycine chromophore.6 Both chromophores are mainly protonated in the ground state but undergo different structural evolution upon photoexcitation, affected by Ca2+ binding at the calmodulin domain. Excited-state proton transfer is identified in the Ca2+-free GEM-GECO1 and Ca2+-free/bound G-GECO1.1, albeit with different local conformational dynamics revealed by the time-resolved Raman spectra prior to fluorescence. The protein chromophores also exhibit a sub-200 fs vibrational frequency shift which may arise from coherent small-scale proton motions. These new structural dynamics insights from FSRS, aided by femtosecond transient absorption, normal mode calculations and molecular dynamics simulations, demonstrate the unique resolving power of FSRS to unravel the governing structure-function relationship of fluorescent protein based biosensors for Ca2+ imaging.