Physical Chemistry
- B.Sc. Sheffield University (1966) - Haworth Medal winner
- Ph.D. University College London and the Royal Institution of Great Britain (1969)
- Member of Technical Staff, Bell Labs, (1969-71)
- IBM Research Fellow, Pembroke College, Oxford (1971-73)
Conformational Relaxation in Isolated Molecular Clusters
When molecules become electronically excited, the rearrangement of electronic charge can precipitate many types of relaxation processes. To probe details of such events, one can employ isolated molecular clusters consisting of only a few hydrogen-bonded molecules. One particularly interesting case is the cluster involving a Coumarin 151 molecule bonded to a water dimer. Two different structures have been identified in the ground state, as shown below.
Recent experiments have shown that the species shown on the left here is unstable in the excited state, and relaxes to that shown on the right on a picosecond or nanosecond time scale depending on the available energy. This corresponds to movement of the water dimer by ~10Å from one side of the molecule to another, following and yet the activation energy is only 60 cm-1. Such conformational changes have been studied by a combination of fluorescence and infrared double resonance techniques in conjunction with ionization and mass resolution.
Hydrogen-Bonded Molecular Dimers
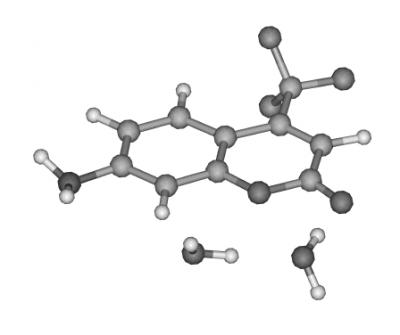
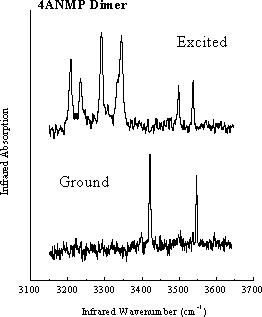
Hydrogen-bonded dimers present important opportunities to study short-range intermolecular interactions, including modification of the electronic structure, and corelated proton or hydrogen atom transfer. Molecules such as the dimer of 4-Amino-N-methylphthalimide, shown here, reveal dramatic changes in their infrared spectra between the ground and excited states. The simple ground-state ground-state infrared spectrum reflects the high symmetry of the ground state. On the other hand, the much more complex excited-state spectrum shows evidence for a loss of symmetry resulting from changes in the acid-base properties of NH2 and >C=O groups, which may result in proton transfer across the intermolecular hydrogen bonds. These types of strongly bonded dimers are different from many other dimer systems studied so far, because both their electronic and vibrational spectra are highly structured, despite the large increase in binding energy upon electronic excitation. Femtosecond-domain experiments are planned, to follow in real time the changes in vibrational spectra, which will provide further insights into the reasons for their complexity in the excited state.
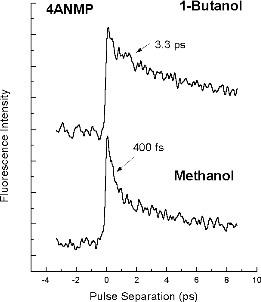
Ultrafast Electronic Relaxation of Hydrogen-Bonded Molecules Studied by Femtosecond Pump-Probe Spectroscopy
Femtosecond pump-probe experiments allow us to observe the time evolution of the first events following pulsed laser excitation, including motions in the first coordination shell of a hydrogen-bonded molecule in fluid solution. New pump-probe experiments involving the detection of ultrashort-lived fluorescence have explored spectroscopic changes of aminophthalimide molecules in hydrogen-bonding solvents on a time scale more than 10 times faster than existing data for fluorescence Stokes shifts. Both fluorescence upconversion and pump-probe methods are being used to investigate ultrafast energy transfer processes in complex molecules, in collaboration with the Regional Laser and Biomedical Technology Laboratories at Penn.