Organic Chemistry, Natural Products Chemistry, Heterocyclic Chemistry
- B.Sc. Simmons College (1949)
- M.Sc.; University of Pennsylvania (1950)
- Ph.D. University of Pennsylvania (1953)
- ACS Philadelphia Section Award (1972)
- ACS Garvan Medal (1978)
- American Cyanamid, Faculty Award (1984)
- American Institute of Chemists, Scroll Award (1985)
- Member of Sigma Xi, Sigma Delta Epsilon
- Member of ACS
- Philadelphia Organic Chemist's Club Award (1994)
- ACS Henry Hill Award (1994)
- Fellow of the New York Academy of Science
- Fullbright Lecturer
- Co-author of two books and several chapters
Investigations carried out in our laboratory encompass a wide range of interests in synthetic organic chemistry including heterocyclic and medicinal chemistry.
Current efforts are in the following areas: (1) synthesis and chemistry of five-membered heterocycles and natural products containing such units; (2) synthesis and chemistry of fungal metabolites; (3) synthesis and chemistry of cyclopeptide alkaloids; (4) synthesis of biologically important depsipeptides; (5) synthesis of novel ninhydrins; (6) synthesis of anti- angiogenic agents.
- Utilization of D-ribonolactone and other sugars as precursors in the synthesis of several structurally challenging molecules is currently underway in our laboratory.
- The synthesis of naturally occurring fungal metabolites containing a common hexasubstituted aromatic ring but different side chains such as colletochlorin D, ascofuranone and ascochlorin are another area of interest. The biological activities of those natural products range from high hypolipidemic action to anticancer and antiprotozoan activity.
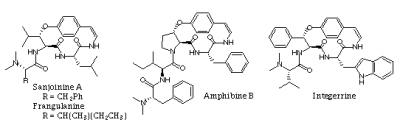
- Cyclopeptide alkaloids are natural products found in many plant families. A broad program aimed at developing methodology for the synthesis of the most commonly found thirteen- and fourteen-membered ring cyclopeptide alkaloids is currently underway. Sanjoin, used in Chinese folk medicine is one of our targets. Other antitumor cyclic peptides provenient from plants, the astins, are also under investigation.
- Didemnins are a new class of depsipeptides isolated from a Carribean tunicate of the family Didemnidae, a species of the genus Trididemnum. These cyclic peptides have shown highly active antiviral and antitumor agents. The synthetic studies carried out in our laboratory have produced synthetic and spectral evidence for the absolute configuration of the asymmetric centers of the hydroxyisovalerylpropionyl (HIP) unit of the macrocycle, thereby requiring a revision of the original stereochemistry. The stereocontrolled total synthesis of these natural products has already been accomplished. The synthesis of several beta-turn mimics and constrained analogs are under investigation. Because of a broad program to develop efficient synthetic routes to the didemnins, other cyclodepsipeptides have been chosen as the next targets. The choice of these compounds was not only based on their relationship to didemnins but also on previous synthetic studies of products originating from polyketide biosynthesis, and earlier investigations of carbohydrates.
- Novel ninhydrins are being synthesized as reagents for the detection of amino acids.
- We have found that sulfated beta-cyclodextrin mimicked heparin advantageously. This effective synthetic product is of utmost importance in the control of angiogenesis and has other important applications in medicine. This recent discovery uncovers a new class of anti-angiogenic agents, consisting of a hydrophilic carrier and a hydrophobic angiostat, and offers a unique opportunity for the development of chemical technologies which will have important applications in the bio- and medical sciences. We are therefore continuing these studies with several goals in mind. We are investigating new and more effective carriers, we are designing single species that contain both the angiostat and carrier, and we are looking for new and more effective angiostats.
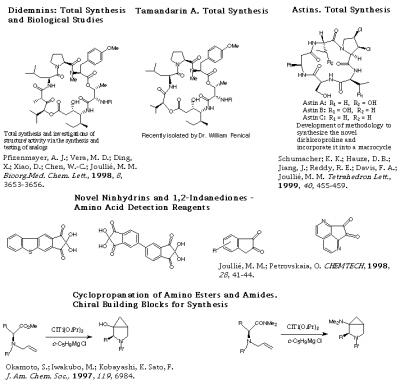
B. Liang, et al. "Total Syntheses and Biological Investigations of Tamandarins A and B and Tamandarin A Analogs." J. Am. Chem. Soc. 2001, 123, 4469-4474.
D. Xiao et al. "Total Synthesis of a Conformationally Constrained Didemnin B. Analog." J. Org. Chem., 2001, 66, 2734-2742.
D. Ahuja et al. "Inhibition of Protein Synthesis by Didemnin B: How EF-1a Mediates Inhibition of Translocation." Biochemistry, 2000, 39, 4339-4346.
D. Ahuja, et al. "Inhibition of Protein Synthesis by Didemnins: Cell Potency and SAR." J. Med. Chem., 2000, 43, 4212-4218.
B. Liang, M.D. Vera and M.M. Joullié. "Total Synthesis of [(2S)-Hiv2] Didemnin M." J. Org. Chem., 2000, 65, 4762-4765.
B. Liang, P.J. Carroll and M.M. Joullié. "Progress Toward the Total Synthesis of Callipeltin A (1): Asymmetric Synthesis of (3S,4R)-3,4-Dimethylglutamine." Org. Lett. 2000, 2, 4157-4160.
P. Portonovo et al. "First Total Synthesis of a Fluorescent Didemnin," Tetrahedron, 2000, 56, 3687-3690.
B. Cao, D. Xiao, and M.M. Joullié. "Synthesis of Bicyclic Cyclopropylamines by Intramolecular Cyclopropanation of N-Allylamino Acid Dimethylamides." Org. Lett., 1999,1, 1799-1801.

